[Surface treatment] About passivation treatment

Passivation treatment is a common process in the industry. Its main purpose is to add a protective layer to the metal surface, improving the corrosion resistance of the workpiece and extending the product's lifespan. It is a very important surface treatment process.
This is because when metals are exposed to air and water, they are easily oxidized and corroded. This can have adverse effects during the production process or at the final stage of product manufacturing. Therefore, protecting the surface of metal components is a primary concern. Metal passivation provides a thin and uniform oxide layer, increasing corrosion resistance and extending the lifespan of parts.
passivation process methods
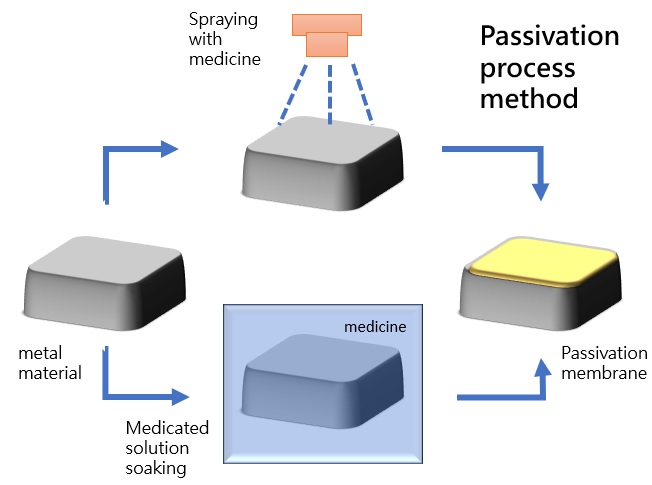
Chemical Immersion
Immersion involves placing the workpiece in a tank containing a chemical solution. Because immersion allows for simultaneous treatment of all workpiece surfaces, it provides more comprehensive corrosion resistance.
Chemical Spraying
Chemical solutions are sprayed onto the surface of the parts using specialized equipment. This process is crucial for the handling of the chemical solution and the surrounding environment; therefore, proper pre-treatment is essential. Alternatively, manual coating can also be used, which can be effective for surface passivation. However, this method is unsuitable for complex workpiece surfaces and is only suitable for relatively simple and smooth surfaces.
Commonly used passivation solutions
Acid passivating agents:
Nitric acid: Commonly used on stainless steel, nickel alloys, etc., it effectively removes impurities and forms an oxide film.
Citric acid: A more environmentally friendly option, often used as an alternative to nitric acid passivation.
Phosphoric acid, sulfuric acid, hydrochloric acid: Can also be used in some acidic passivation processes.
Alkaline passivating agents:
Sodium hydroxide: Commonly used to treat metals such as iron, steel, and aluminum.
Potassium hydroxide: Also a component of alkaline passivating agents.
Other passivating agents:
Ferricyanide: Used for treating steel.
Zinc salts: Used for treating galvanized steel.
Titanium salts: Used for treating titanium and titanium alloys.
Conversion coating passivating agents: Contain various components, such as phosphating, chromate passivation, etc.
Chromate passivating agents:
Hexavalent or trivalent chromium compounds: such as hexavalent chromic acid or trivalent chromium chloride. Mainly used for passivation of aluminum and magnesium, and for sealing pores in certain coatings.
Advantages and disadvantages of passivation process
Advantages
- Residual contaminants can be removed after machining.
- Improved corrosion resistance.
- Reduced contamination during manufacturing.
- Improved component performance.
- Achieves a uniform and smooth surface.
- Easy to clean surface.
Disadvantages
- Passivation is not effective at removing contaminants from welded parts.
- The choice of operating conditions and type of chemical bath is crucial depending on the metal alloy used, and detailed information is required.
- Acidic baths can damage some metal alloys with low chromium and nickel content, and are therefore unsuitable for passivation.
Product Application

